Single Point Scanning
Single point scanning confocal microscopy, also known as Point Scanning Confocal Microscopy or traditional confocal microscopy, is a technique that uses a focused laser beam to sequentially scan a single point or small region of interest within a sample. It provides optical sectioning and high-resolution imaging capabilities by rejecting out-of-focus light.
The key features and principles of single point scanning confocal microscopy are as follows:
1. Laser illumination: A laser beam is used as the light source in confocal microscopy. The laser is typically focused through the objective lens to a diffraction-limited spot on the sample. The laser wavelength is selected based on the excitation characteristics of the fluorophores used in the sample.
2. Pinhole aperture: After interacting with the sample, the emitted fluorescence or reflected light is collected by the same objective lens. Before reaching the detector, the collected light passes through a pinhole aperture. The pinhole blocks the light emitted or reflected from out-of-focus regions, allowing only the light originating from the focal plane to pass through.
3. Scanning mechanism: The laser beam is scanned across the sample in a raster pattern using a scanning mechanism, such as galvanometer mirrors or other scanning devices. The scanning mechanism directs the laser beam to different points within the region of interest, enabling the acquisition of a complete two-dimensional image.
4. Confocal detection: The light passing through the pinhole is directed to a detector, typically a photomultiplier tube (PMT). The detector converts the light signal into an electrical signal, which is then used to construct the image. The signal strength corresponds to the intensity of fluorescence or reflected light at each scanned point.
5. Image reconstruction: The detector output is collected as a series of intensity values corresponding to each point scanned within the region of interest. These intensity values are used to construct a two-dimensional image of the sample. The image can be further processed, analyzed, and visualized using image analysis software.
Single point scanning confocal microscopy offers several advantages:
● Optical sectioning: By selectively illuminating and detecting a single point at a time, the technique provides optical sectioning capabilities. Out-of-focus light is rejected, resulting in sharper and more defined images of the focal plane.
● High resolution: The focused laser beam and the rejection of out-of-focus light contribute to improved resolution in confocal microscopy compared to wide-field microscopy. This enables the visualization of fine details and structures within the sample.
● Versatility: Single point scanning confocal microscopy can be applied to various samples, including biological specimens, thin films, materials, and more. It is a widely used technique in many research fields, such as biology, materials science, and nanotechnology.
However, it is important to note that single point scanning confocal microscopy can be relatively slower compared to newer techniques like spinning disk confocal microscopy or swept-field confocal microscopy. These techniques utilize multiple points of illumination simultaneously to accelerate image acquisition.
Overall, single point scanning confocal microscopy remains a valuable tool for high-resolution imaging and optical sectioning, providing detailed insights into the structure and function of biological and non-biological samples.
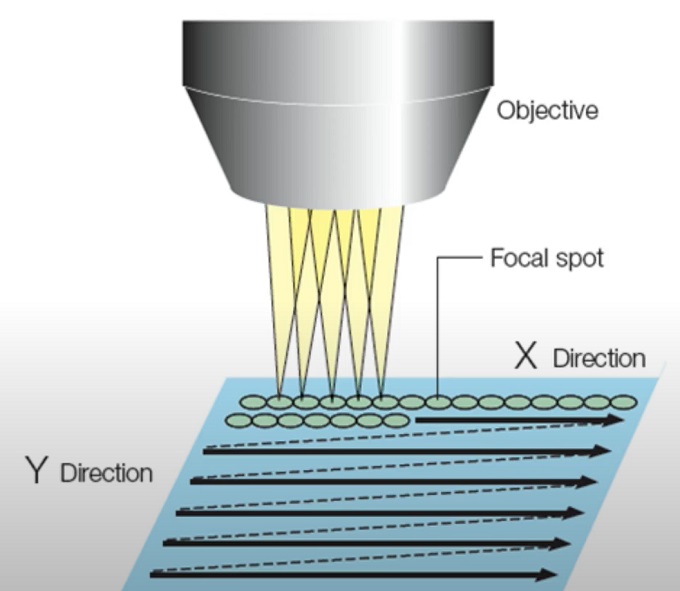
Single point scanning confocal microscopy
Line scanning
Line scanning confocal microscopy is a technique that combines the principles of confocal microscopy with the ability to rapidly acquire images along a line instead of a single point. It allows for faster imaging of dynamic processes within biological samples.
In line scanning confocal microscopy, instead of scanning a focused laser beam point-by-point in a raster pattern, a line of laser illumination is scanned across the sample. This line can be a straight line or a customized pattern depending on the specific imaging requirements.
The key features and principles of line scanning confocal microscopy are as follows:
1. Laser line illumination: A laser beam is expanded and shaped into a thin line using cylindrical optics or a beam-shaping element. This laser line is directed onto the sample through the objective lens.
2. Scanning mechanism: A scanning mechanism, such as galvanometer mirrors or an acousto-optic deflector, rapidly scans the laser line across the sample in a direction perpendicular to the line itself. This allows for the acquisition of image data along the line.
3. Confocal detection: The emitted fluorescence or reflected light from the sample is collected by the same objective lens. Similar to other confocal techniques, a pinhole or a slit aperture is used to block out-of-focus light. The light passing through the pinhole or slit is directed to a detector, typically a photomultiplier tube (PMT).
4. Line detector: Line scanning confocal microscopy utilizes a specialized detector that can capture the fluorescence or reflected light signal along the scanned line. This detector can be a linear photodiode array or a charge-coupled device (CCD) camera.
5. Image reconstruction: The detector output provides intensity values for each point along the scanned line. These intensity values are used to construct a one-dimensional image representing the scanned line. Multiple lines can be sequentially scanned to form a two-dimensional image of the sample.
Line scanning confocal microscopy offers several advantages:
● Rapid imaging: By scanning a line instead of a single point, line scanning confocal microscopy allows for faster image acquisition. It is particularly useful for imaging dynamic processes, such as calcium signaling, membrane dynamics, or neuronal activity.
● Reduced photobleaching and phototoxicity: The rapid scanning of the laser line reduces the dwell time of the laser on any particular region, minimizing photobleaching and phototoxicity effects, making it suitable for live-cell imaging.
● High spatial resolution: Line scanning confocal microscopy can achieve high spatial resolution along the scanned line. This enables detailed visualization of fine structures and features within the sample.
Line scanning confocal microscopy is a valuable technique for studying dynamic biological processes that require fast imaging, as well as for obtaining high-resolution images along a line of interest. It has found applications in neuroscience, cell biology, developmental biology, and other fields that involve the visualization of rapid cellular events.
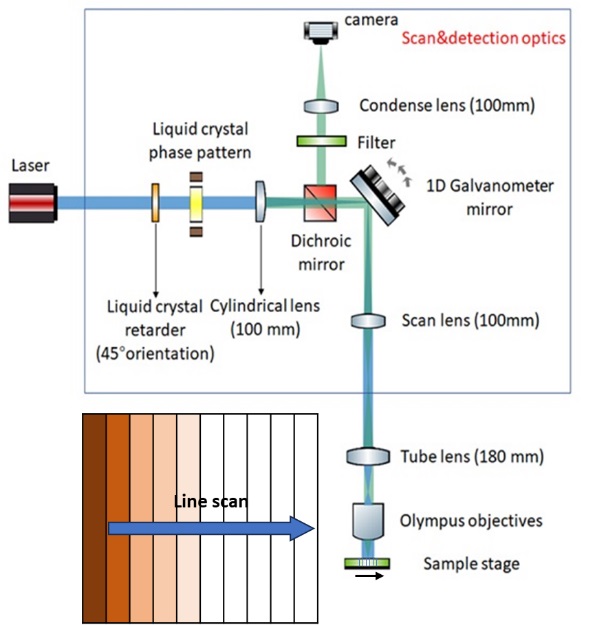
Line scanning confocal microscopy
Spinning disk
Spinning Disk Confocal Microscopy is an imaging technique that combines the principles of confocal microscopy with a spinning disk containing multiple pinholes or slits. It allows for repid and efficient imaging of biological samples while providing optical sectioning and improved signal-to-noise ration sompared to traditional wide-field microscopy.
The key features and principles of spinning disk confocal microscopy are as follows:
1. Spinning disk: The heart of spinning disk confocal microscopy is a spinning disk containing multiple pinholes or slits arranged in a pattern. The disk is placed in the optical path between the sample and the detector.
2. Laser illumination: Laser light is focused onto the spinning disk, which then creates a pattern of illuminated spots on the sample through the pinholes or slits. The pattern is continuously changing due to the rotation of the disk.
3. Confocal detection: The emitted fluorescence or reflected light from the sample is collected by the same objective lens that provides the laser illumination. The light passes through the spinning disk, and only the light passing through the open pinholes or slits reaches the detector.
4. Optical sectioning: The spinning disk rapidly scans multiple illuminated spots across the sample. As the spinning disk rotates, each spot briefly overlaps with a pinhole or a slit, allowing light emitted from the corresponding focal plane to pass through. Light emitted from out-of-focus planes is blocked by the pinholes or slits, resulting in optical sectioning.
5. Detector: The light passing through the pinholes or slits reaches a detector, typically a sensitive camera or a photomultiplier tube (PMT). The detector captures the light signal, converting it into an electrical signal that can be processed into an image.
6. Image reconstruction: The detector output is used to construct a two-dimensional image of the sample. The image represents a composite of the individual illuminated spots collected during the spinning disk rotation.
Spinning disk confocal microscopy offers several advantages:
● Enhanced imaging speed: The spinning disk allows for simultaneous illumination of multiple points on the sample, resulting in faster image acquisition compared to point scanning confocal microscopy. This makes it suitable for imaging dynamic processes and fast cellular events.
● Reduced photobleaching and phototoxicity: Since the exposure time of each illuminated spot is brief, spinning disk confocal microscopy reduces the risk of photobleaching and phototoxicity, making it ideal for live-cell imaging.
● Improved signal-to-noise ratio: The use of multiple pinholes or slits in the spinning disk, combined with the rejection of out-of-focus light, leads to improved signal-to-noise ratio and higher image contrast compared to wide-field microscopy.
Spinning disk confocal microscopy is widely used in cell biology, developmental biology, neurobiology, and other fields where fast and high-resolution imaging is required. It provides an effective balance between imaging speed and optical sectioning capabilities, allowing researchers to capture dynamic cellular processes with enhanced spatial resolution.
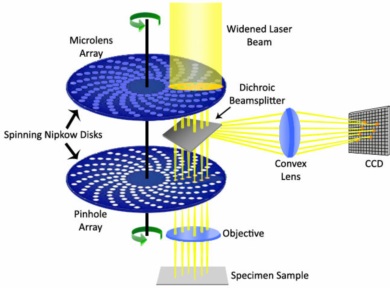
Spinning disk confocal microscopy
Disclaimer: The picture comes from the internet, if there is any infringement, please contact to delete the post.