 | Features & Benefits
✔ High-speed acquisition ✔ Strong optical sectioning ✔ Single molecule imaging ✔ Multi-color fluorescence ✔ High sensitivity ✔ Large field of view ✔ Low photobleaching and phototoxicity ✔ Super-resolution ✔ Intuitive software | Brochure Download
-English Version PDF -中文版 PDF
Request for Demo or Sample measurement Please contact us:
info@simtrum.com
|
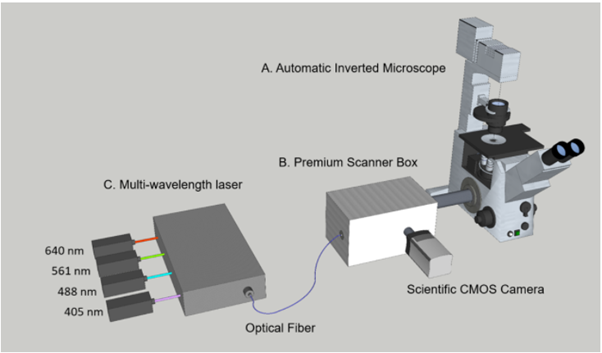
LSFMM is a high-speed, high-contrast multi-dimensional imaging platform capable of three key imaging advantages. At its core is a focal modulation module for high-contrast imaging. It significantly reduces the background signal caused by multiple scattering and effectively picks up the high-resolution signal related to the ballistic excitation light. Consequently, the signal to background ratio and the spatial resolution can be maintained to a deeper penetration depth, which is about two to four times deeper than conventional confocal microscopes. High acquisition speed is the second feature of LSFMM. Capturing at speeds at least 100x faster than conventional confocal technology, LSFMM is the optimal solution for live cell and tissue imaging, providing low phototoxicity and photobleaching, or perfect for fast volume acquisition of fixed samples and even small live animals.
The third characteristic is large field of view (FOV) available. Our scientific CMOS camera can offer up to 5.5 Megapixel sensor, yielding the largest available field of view with 60x objectives(0.36 mm) and 40x objectives(0.54 mm). Maximizing view in fluorescence microscopy is of increasing relevance across a wide range of applications, including high content screening of large fields of cells, imaging of the developing embryo, neuron mapping and tissue imaging.
Core technology 1: Focal modulation The combination of electro-optic modulator and self-designed liquid crystal phase plate only modulates the intensity of signal fluorescence at the focal point, while stray light and background light are not modulated. Through the principle of modulation and demodulation technology, a strong focal area signal is extracted, thereby increasing the signal-to-noise ratio and signal-to-background ratio of the image by 20-30dB, as well as the imaging depth |
| Core Technology 2: High-speed line scanning
The microscope system uses a cylindrical lens to form a line focus, thus replaces the traditional confocal point scanning method with line scan, which greatly increases the scanning speed and thus the imaging frame rate. Traditional point-scanning confocal microscopes generally take a few seconds to acquire a large-field fluorescence image; under the same field of view, line scanning can collect dozens of images per second, which can capture high-dynamic biological images of cells or tissues. |
|
| 
|
Product Model Comparison:
Hardware Feature | Hardware Feature | LSFMM Premium
(FP-10-P) | LSFMM Advance
(FP-10-A) | LSFMM Standard
(FP-10-S) |
High-speed line-scan laser confocal imaging | Up to 600 fps for fast cell dynamics At least 100 x faster than conventional confocal | ✓ | ✓ | ✓ |
FMM image contrast enhancement | Imaging deeper in cells and tissue At least 2-4 times deeper than conventional confocal 20-40 dB enhancement in image signal-to-background ratio | ✓ | ✓ | ✓ |
Large field of view | Capture more in a single image Matches large sCMOS sensors | ✓ | ✓ | ✓ |
Low noise level | Acquire noise-free images with weak fluorescence | ✓ | ✓ | - |
16-bit dynamic range | Capture both weak and bright signals without saturation | ✓ | - | - |
Multi-color fluorescence imaging | Choice of 4 wavelengths up to 640 nm Any two colours simultaneously - match penetration depth of two labelled targets instantly | ✓ | Optional | Optional |
Super-resolution | Acquire higher resolution images than the diffraction limit | ✓ | - | - |
Z-motorized stage | Acquire volumetric images automatically | ✓ | ✓ | - |
Technical Data:
| LSFMM premium
(FP-10-P) | LSFMM Advance
(FP-10-A) | LSFMM Standard
(FP-10-S) |
Laser combiner | 405nm, 488nm,561nm, 640nm (original configuration);
Laser wavelengths are optional according to user requirement. | 488nm, 561nm (original configuration);
Laser wavelengths are optional according to user requirement. | 488nm (original configuration);
Laser wavelengths are optional according to user requirement. |
Laser power | 50 mW maximum for combined wavelengths |
Frame rate | 42 fps (2560 x 500 pixels)
210 fps (2560 x 100 pixels)
Fast scan mode:
168 fps (2560 x 500 pixels) | 8 fps (1936 x 500 pixels)
40 fps (1936 x 100 pixels)
Fast scan mode:
32 fps (1936 x 500 pixels)
160 fps (1936 x 100 pixels) | 60 fps (1280 x 1024 pixels)
600 fps (1280 x 100 pixels) |
Image resolution | 100 x 100 pixels to 2560 x 2560 pixels | 100 x 100 pixels to 1936 x 1936 pixels | 100 x 100 pixels to 1280 x 1024 pixels |
Image format | 8/16 bit | 8/12-bit | 10 bit |
Noise level | 0.9e- | 6.2e- | 12e- |
Noise level(count in photons) | 1.5 photons | 8.8 photons | 20 photons |
FMM modulation | line-by-line | frame-by-frame |
Lateral resolution | 1.2-1.4 fold over optical diffration limit | Optical diffration limit |
Number of fluorescence channel | >4 | >2 | >1 |
Microscope stage | Fully-motorized and automated stage with piezo z scanner | Semi-motorized stage with piezo z scanner | Mechanical stage with focal spiral(can upgrade to piezo z scanner) |
Applications
LSFMM is a novel microscopy method that has been developed to achieve a deeper penetration depth on the basis of confocal microscopy. Equivalently selective excitation is achieved by modulating the light intensity at the focal point only. Fluorescence emission or backscattered light are collected and demodulated. As a result, only focal signals are decoded and thus significantly reducing the background signals. Specially, we add a cylindrical lens to generate a line-focus at the sample and achieve line-scan imaging. Therefore, the imaging acquisition speed is improved significantly compared with conventional point-scan confocal microscopes.
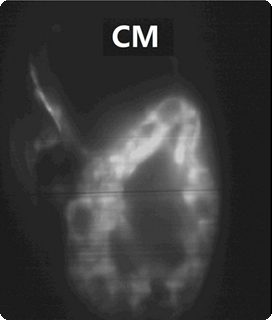
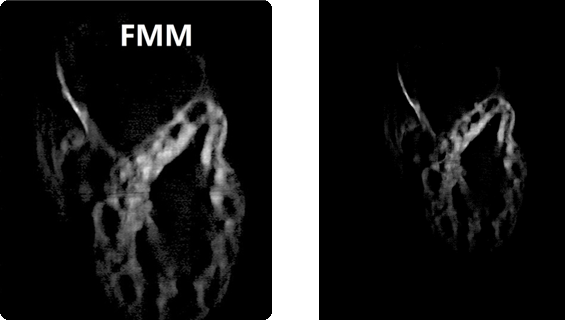
3-day post-fertilized zebrafish heart labelled by EGFP
| 
|
| Conventional confocal microscoe | Our focal modulation microscope |
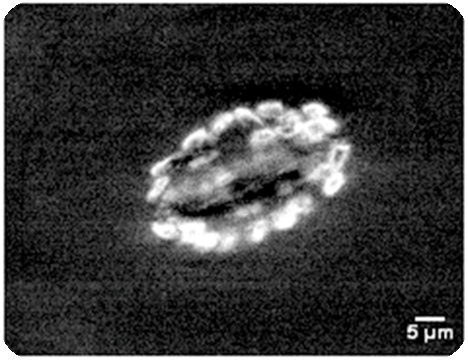
Autofluorescence of leaf chloroplasts
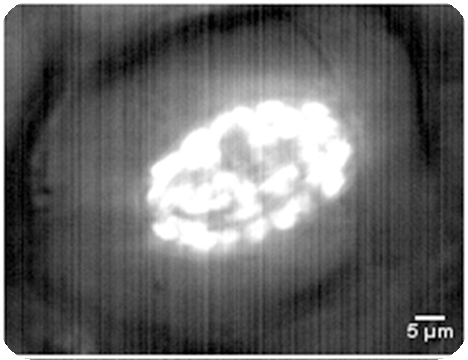
| 
|
| Conventional confocal microscoe | Our focal modulation microscope |
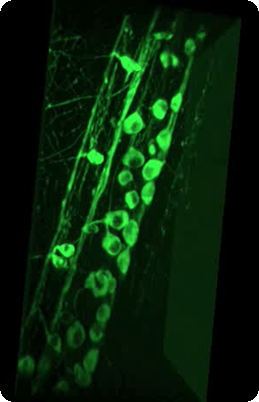
3D rendering of zebrafish neurons from spinal cone labelled with GCamp6

| 
|
Conventional confocal microscoe | Our focal modulation microscope |